In December 2023, the Food and Drug Administration (FDA) approved two new gene therapies that can cure sickle cell disease. The upsides: for the first time in 30 years, the therapies offer a chance at a pain-free life, and they will likely be covered by insurance. The downsides: the treatment takes months to undergo and it requires chemotherapy that comes with side effects.
The news is being celebrated in Aurora, which is a hub for sickle cell care in Colorado (where about 400 people are living with the disease) and for neighboring states.
“This is an exciting time for sickle cell … and this has radically changed our conversations that we have with patients,” said Chris McKinney, a hematologist at Children’s Hospital Colorado in Aurora who specializes in caring for patients with the disease. “We’re very relieved to be able to have additional options for our patients who have struggled for a long, long time with very few good options for over 30 years.”
Before the FDA’s approval, McKinney, 40, had been caring for dozens of patients regionally. He was also running clinical trials testing a similar, yet-to-be-approved version of the therapy on patients willing to take the chance. Through his work, he was able to see gene therapy effectiveness: nine in 10 of his patients who participated in clinical trials are now in remission.
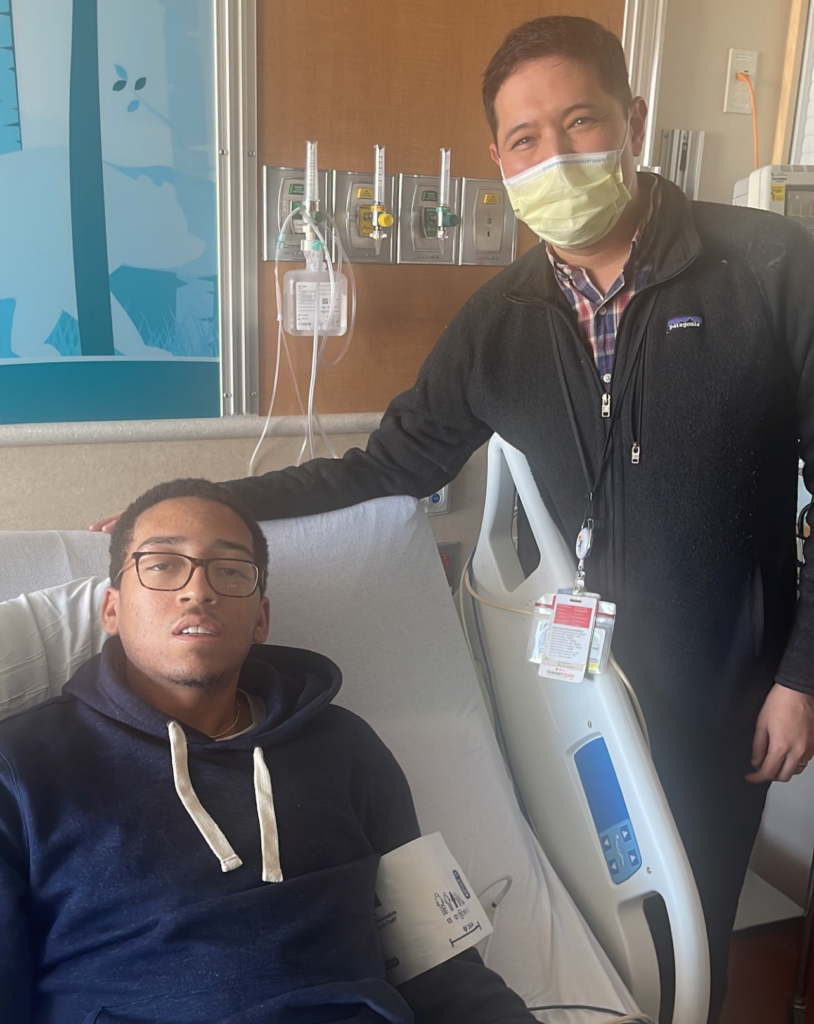
Xavius Hymes, a 28-year-old Little Rock, Ark., native and aspiring doctor, is one of those patients. As a child, he said living with the disease wasn’t so bad, but the pain it caused worsened by the time he was in college. The disease can lead to organ failure and anemia, but pain – sometimes emergency-room level – is the most common symptom.
“Growing up, I really didn’t have as many crises with sickle cell,” Hymes said in a recent virtual interview from Arkansas. “When I was getting older, I started having more and more sickle cell crises. But I’ve always had this dream of becoming a physician, and as I got older, the more crises I had, the harder it made that journey.”
A report from the Centers for Disease Control and Prevention (CDC) stated the origins of the pain: “Sickled cells traveling through small blood vessels can get stuck and block blood flow throughout the body, causing pain. A pain crisis (vaso-occlusive episode or VOE) can start suddenly, be mild to severe, and can last for any length of time.”
“If you got hit by a bus and survived, all that excruciating pain that you would feel, that’s how I’d describe it. I’d spend a week, two weeks, in the hospital, and then you have to recover, that’s another week, and I’d miss two weeks of class,” Hymes said when explaining what living through a pain crisis has been like for him.
So, he looked for a solution by doing some online research that led him to McKinney’s work.
“We shot a few emails back and forth and it just felt like the right place to be … I knew that the life I wanted and how it was going couldn’t really co-exist,” he said. “Leading up to around the time I reached out to Dr. McKinney, I started to have crises almost every other month or sometimes even every month.”
After he and McKinney developed an email relationship, Hymes came to Colorado for some blood work and testing and discovered he was a match for the trial.
“Once it was determined that I could, I became a little more hopeful in the journey,” said Hymes, who didn’t dwell on the challenges of that journey, and instead honored the people who helped him through it: his mother, his girlfriend, and his grandparents — all of whom came to visit him in Colorado, while he spent months coping with the complicated procedure.

“We evaluate the patient to ensure their organs are healthy enough to tolerate the gene therapy process; we then start putting them on chronic transfusions where they get a monthly transfusion to decrease how much sickle hemoglobin they have," explained McKinney. "We give them medications to help them release their stem cells from their bone marrow into their blood and we hook them up to machines to collect those stem cells.”
It’s those cells that then get the gene therapy, they are edited to stop forming the sickle shape.
“And that may take several months to get enough stem cells ... once we’ve collected those stem cells, we have to send those stem cells off to manufacture and to change the genes so that they make non-sickling hemoglobin. And that process can take about 12 weeks,” McKinney said.
Patients then return for high doses of chemotherapy, which gets rid of the old bone marrow and makes room for the new.
“Once they get that chemotherapy, we then infuse the stem cells, and it can take about four weeks for those cells to grow,” McKinney said. The patient remains in the hospital while the new stem cells grow. Upon discharge, they have years of follow-up monitoring. “Ultimately, patients who receive gene therapy need to be followed for about 15 years after the infusion to make sure they’re doing well."
For Hymes, things went according to plan. Because he was from out of town, he had to stay in Colorado for about six months in total. He said part of it was “to have my edited genes put back in my body, and that’s when I would have the chemo and stay in the hospital for an extended period of time.”
Recovering from the chemotherapy, Hymes said, was one of the hardest parts.
“I was still having to deal with the chemo and all the problems that it can cause. It’s just this really bad taste you could have from the chemo and it could knock out your taste and you have a sore throat ... I guess I’m a big eater, so food was the important part to me. But I drank a lot of Ensures just recovering from the chemo,” said Hymes.
Now, two years since his journey began and one year after the transplant, Hymes said he feels like a new man.
“It was a total change in my energy level and how I was able to just go about life! I didn’t have to worry about having pain crises every other day or every month,” said Hymes.
Regarding his pain crisis, Hymes said, “It’s almost non-existent at this point. I don’t say ‘almost non-existent.’ It’s been non-existent at this point.”
Like about 90 percent of McKinney’s other trial participants, it’s all over now.
“I haven’t had any crises since I had the stem cell transplant, and sickle cell-related problems since the transplant. So I would say it has been extremely beneficial in my life,” said Hymes. He said it's also been good for his mother.
“I only seen my mom cry one or two times,” Hymes explained. One time was seeing him struggle with the disease, the other time, Hymes said, was seeing him leave it behind.
The two FDA-approved treatments are slightly different from what Hymes received – but they work the same way, editing cells, and then returning them to the patient.
Now that the drug therapy has been approved, another component of McKinney’s job is to let the rest of his patients – the ones who weren’t part of a trial – know about it. Some of those who weren’t in any of his trials experience pain from the disease serious enough to make the therapy worth considering.
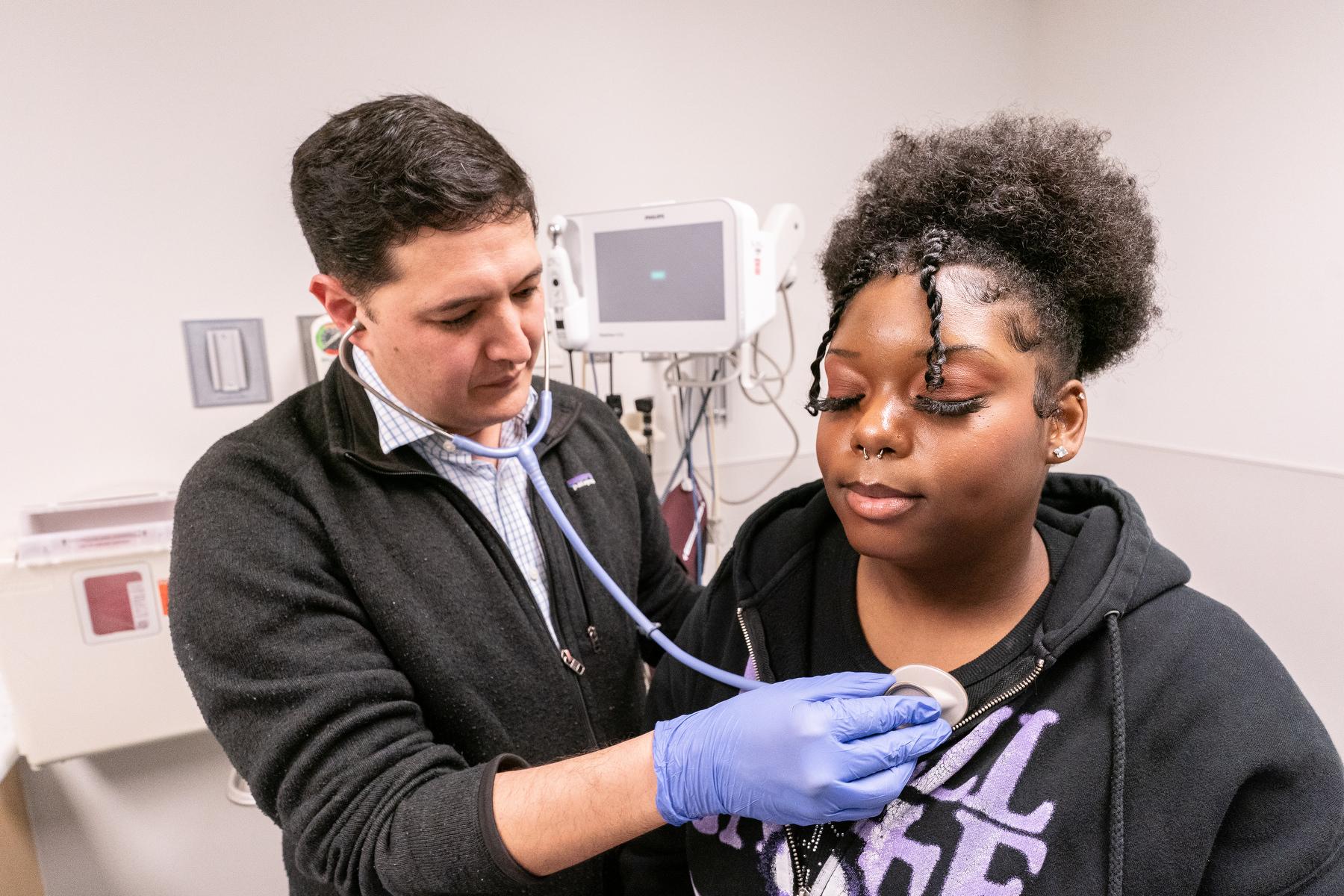
A few weeks after the FDA announced its approval of the drugs in early December, McKinney met with Mia Hilton, a 20-year-old esthetician from Green Valley Ranch in Denver. Hilton has been McKinney’s patient for the past 10 years. Vivacious and upbeat, she spent most of the meeting making funny comments. After a quick overall exam, he asked if she’d heard about the newly approved gene therapies.
“They are meant to be curative,” he explained. “You’d no longer have sickle cell after administration of them.”
For her, that would be a big change. Like Hymes, sometimes, her pain got bad enough to go to the ER, where she’d sometimes receive intravenous drugs.
“Usually a pain crisis feels kind of like burning or stabbing a little bit. It can feel pretty hot internally in your muscles. I'll take a Benadryl and then they'll give me morphine through my IV, and that usually works,” said Hilton. If not, she gets a nasal dose of fentanyl. In the ER, medical staff monitor her progress.
“They usually do a chest X-ray to make sure that I'm not going to have a chest crisis,” explained Hilton. And after a few hours, if the pain was under control, she’d get discharged. “I'll have somebody come and pick me up from the ER just because of the heavy medicines, they don't want me to drive.”
Sometimes, when she didn’t bounce back, she would be admitted from the ER into the hospital.
“Usually my stay can range from three to seven days, so it does get pretty strenuous,” she said.
She told McKinney that she’d heard some pieces of information about the new gene therapies, but wanted to know more. In a cautious, gentle voice, he told her, “The side effects of the chemo could cause infertility and hair loss.”
Hilton had a quick answer to that.
“I’ve never wanted babies,” she said, “So it’s a free form of birth control.”
“I'm very into make-up and hair, so I already have 40 wigs in my closet,” the bubbly woman added while already wearing eyelashes nearly an inch long. When McKinney mentioned that painful sores could form in her mouth, she didn’t care about that either.
“I bite my jaws a lot, so I’m already there. I bit my tongue and my jaw four times in the elevator on the way up here,” said Hilton.
Her reservations focused on the time she’d lose working, and concerns about whether her case was severe enough to warrant the sacrifice.
“I love science, so to hear that you're going to take something out, fix it, and put it back, it's like, ‘Oh my gosh, that's kind of cool.’ Personally, I would definitely look into it, but ... I don't know if I would have the energy to do that. You know what I mean? I would be pretty much down to do it because I feel like in the long run, it could really help because having sickle cell, if you're not on top of it and you're not maintaining it, it can really slow you down ... So to get rid of crises altogether, that would be pretty ideal,” said Hilton.
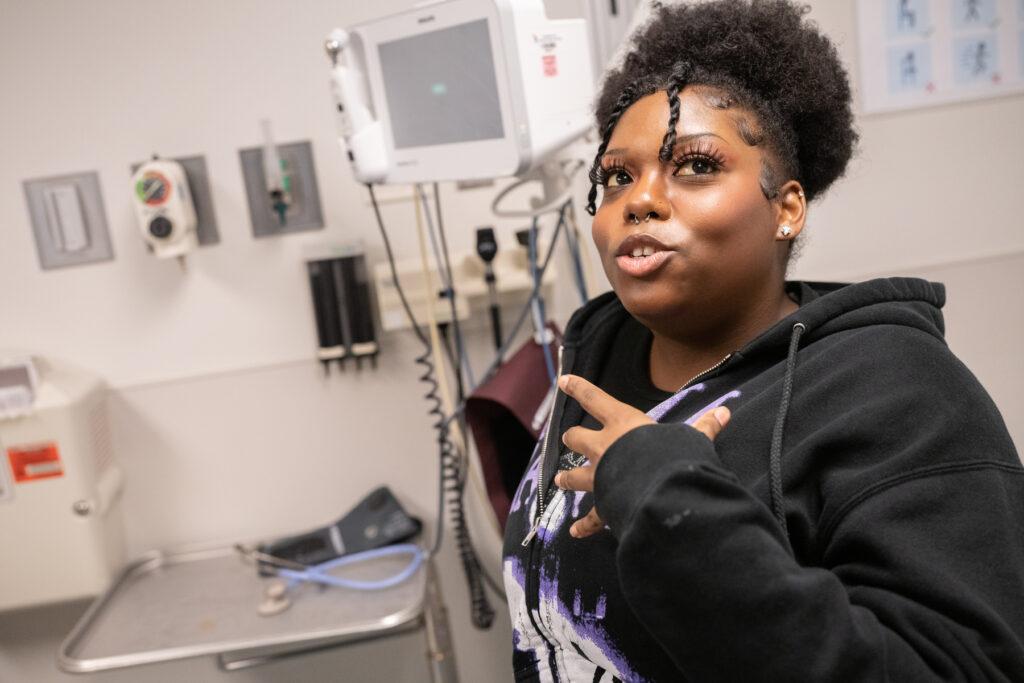
Although the side effects didn’t bother Hilton, the cost could if her insurance won’t cover it. One therapy costs $2 million, and the other is $3 million. But, according to Dr. David Rind, chief medical officer at the Institute for Clinical and Economic Review, a nonprofit that evaluates the prices and effectiveness of medications, that price wouldn’t come out of the patient’s pocket. He said he expects insurance companies to cover the gene therapy.
“I don’t think any manufacturer would charge a price like that thinking only the patients who can afford it will get it,” Rind said. “I think the assumption is that insurance will pay for this ... I think this will be paid for, for almost anyone who wants it.”
The rationale for why insurers should cover the gene therapies relates to slavery, he said.
“The reason there is sickle cell in the U.S. is because we brought Africans over as slaves,” Rind said. He explained that the disease is a gene mutation that fights malaria, a disease common in Africa. “That’s why the people who are here have sickle cell …. This is a population obligation in the United States to get this right.”
Hymes has a sense of obligation, too, to other people born, like him, with the disease. Now that he has gotten rid of his crises as a clinical trial participant, what he thinks would be ideal is to become a doctor caring for people with sickle cell.
“My entire life, I’ve been extremely interested in hematology-oncology, of course, because that is what [sickle cell disease] is grouped as. But lately, I’ve been interested in emergency medicine as well, because I know a lot of times we get seen on the front end in the emergency room, and that’s where a lot of chronic pain patients get perceived as opioid-seeking,” said Hymes.
He is now participating in an academic program for aspiring medical students while waiting to hear if he’s been accepted to medical school. Once he has completed his medical degree, he said, he knows he’d have the sensitivity to understand a sickle cell disease patient’s experience as real, not one of faking pain in the ER. “
So either helping on that side or helping in the hematology and oncology area is what I’m interested in. If I could do both or figure out a way to help all across the board, then I’d probably choose that, too,” said Hymes.
McKinney looks forward to offering his patients the choice of a different ending.
“Now, when those families come in for their first visit with us to talk about what it’s going to look like for their child to live with sickle cell disease, we can at least provide them some hope that this is something that is very, very treatable,” McKinney said. “Something that if they’re having a lot of problems related to this, that we can potentially cure and get rid of.”









